Keyboard Shortcuts
Likes
- XRF
- Messages
Search
XRF Wiki
Welcome to the XRF Wiki. This Wiki is a repository of information contributed by members of [email protected]. Members can view and edit the pages. The pages are currently not viewable by the public. The pages below represent a rough overview of the technology and techniques associated with X-Ray Florescence Spectroscopy as well as serving as a place for members to organize the spectra that they have contributed to the forum.?
As a starting point, the pages will be populated by information taken from member posts from the past few years - with references/attribution. Perhaps in time, these pages can be edited by members to make them more complete and less choppy. Members can also edit this page, making the structure of the content more organized.?
Pages can contain information about a particular topic, links to relevant resources (such as manuals, research papers, etc.), links to relevant forum discussions on the topic, book titles, or anything that might be useful.
What follows is very much a work in progress.
?
Adding to the Wiki
For those unfamiliar with the Wiki phenomenon, it is basically a user editable encyclopedia. The idea is that there are pages with different articles, which the user can read and also choose to edit if desired. If a piece of information is incorrect, a link outdated, or the presentation choppy or unclear, the user can just click "Edit Page" at the bottom and then fix the mistake. There is also a "Page History," so if you a user were to make a mistake or maliciously destroy a page (which wouldn't happen here) an editor can come along and restore the page to a previous version.?
If you have used MediaWiki (the engine that runs behind the scene of Wikipedia.org) you will be familiar with a certain style of creating an article. There are tags that one uses to change the formatting of the parts of the article. The Wiki on Groups.io uses a different background engine and so the standard MediaWiki syntax does not apply here. Rather, the page is edited using the same tools as one would use to compose a message on the forums. If you are a programmer or someone who likes using the tagged syntax directly, you can access the underlying page source code, be activating the advanced editing toolbar (the icon with three lines on the far right of the basic toolbar) and then clicking on the source code icon <> on the far right of the Advance Editing Toolbar. You will immediately notice that the underlying source code is HTML. Most things can be done just by using the various icons, but advanced features like table can only be implemented by manually writing the source code. (Although there are website available that will generate the source code for you through a graphical interface.)
Here are some useful resources from Groups.io:?
Wiki Guide for Users and Editors
Message Composition Tips and Tricks
Overview of XRF
X-Ray Florescence vs. X-Ray Diffraction
?
Hardware
This section describes the hardware used for XRF. We will describe the basic theory of operation of each stage in the processing tool chain and then present different commercial systems that are available as these systems are often integrated together.
[Editor note: General theory needs to be in an independent page separate from specific manufacturer information.
[Editor note - question: Should this be arranged by category, e.g detectors, preamps, etc or by manufacturer with the manufacturers product line on one page. Perhaps it's best to put a list of products with theory but the details of the products on a manufacture page...]
Detectors?- what's available, theory of operation, tradeoffs
Detector Cooling?- keeping detectors cool to avoid thermal noise and keeping the TEC from overheating
Preamps?- theory of operation, brief summary of what out there (and what not to do - such as trying to use a PMT preamp for a SiPIN diode...)
Pulse Processing Theory - high level overview of the stages of going from detector pulse to channel peaks on the computer
Commercial Systems - many commercial systems are integrated so it seems to make sense to present, for example all Amptek products together. [question: are their other affordable integrated systems besides Amptek?]
Amptek XRF System
Activation Sources - what do you use to make the sample fluoresce (that's within the amateur budget and doesn't require special licensing)
Software
- Device Control Software - what do you use to collect the data from the detector system
- XRF Analysis Software - what do you use to analyze the data, especially for quantitative measurements (composition percentages, thin film measurement)
- Free Software Tools
Note: there may be software packages that possess some or all of these characteristics.
Materials Spectra
Metals Spectra
Historic/Archeological Spectra
Reference Materials Spectra
Household Objects Spectra
?
Resources
Videos
Articles
?
Non-XRF Techniques
Gamma Spectroscopy
Raman Spectroscopy
FTIR - Fourier Transform Infrared Spectroscopy
Atomic Emission Spectroscopy
Inductively Coupled Atomic Emission Spectroscopy
Spark Emission Spectroscopy
Atomic Absorption Spectroscopy
LIBS - Laser Induced Breakdown Spectroscopy
Photoacoustic Spectroscopy
?
|
Re: Yttrium Ore XRF
开云体育Beam current = 0.0 uA (8 pellet SDD CAP was excitation source). ?
On 2019/10/04 06:41 PM, Dude wrote:
|
||
|
Re: Yttrium Ore XRF
开云体育You’ll also need a micro focus xray source to put on that bill as well. Dud ? From: [email protected]
[mailto:[email protected]] On Behalf Of Charles David Young
Sent: Friday, October 4, 2019 2:33 PM To: [email protected] Subject: Re: [XRF] Yttrium Ore XRF ? I got a quote from Amptek that the detector is only $4000 but the power supply and preamp is another $6000.? If you know what to look for on ebay the system can be had for less, of course. ? Charles ? On Fri, Oct 4, 2019 at 1:57 PM David Eckhardt <davearea51a@...> wrote:
|
||
|
Re: Yttrium Ore XRF
开云体育Try putting the plot for the Y scale in log space, it should ?help sort out the big from the small.? What was your live time and beam current on this shot? ? Dud ? From: [email protected]
[mailto:[email protected]] On Behalf Of GEOelectronics@...
Sent: Friday, October 4, 2019 1:09 PM To: [email protected] Subject: Re: [XRF] Yttrium Ore XRF ? Same sample as first post, same
scan and data, but with the gain turned up to bring up lesser, but still
interesting peak-pairs.
|
||
|
How to excite XRF from a stable element. or: What IS a RAPCAP/ SDCAP/ SDDCAP?
Every atom has electron shells surrounding the nucleus. In its most stable form, an atom will have the same number of electrons in those shells as it has protons in its nucleus.
Every single electron in the orbitals of the same elemental atoms (atoms with same protons #s) will be bound to the nucleus with individual unique binding energy according to that element's scheme .From one atom to the next, as long as they have the same elemental name and therefore the same number of protons in the nucleus, this scheme will be identical. All identical elements will have the identical scheme. No two electrons in a given element will have the same binding energy, and no single electron in any different element's atoms can have the same binding energy as the first element. To recap, every electron in every element has a unique binding energy associated with that element only. Upon application of extra kinetic energy to the atom, the electrons can pick up (absorb) some or all of that energy and some interesting things start to happen. This energy can be from various external sources and can be conveyed into the atom by X-Rays, Gamma Rays, and even charged particles such as energetic electrons or alpha particles. Once an inner electron achieves a higher kinetic energy than allowed by its unique orbital, it will leave that orbital position and take up a higher orbital or even leave the atom entirely, leaving a hole where it was originally. Atoms MUST fill the innermost holes and do so with high priority. When an electron from a higher orbital fills that hole, the amount of energy equal to the binding energy ifs given off as an electromagnetic photon, which we call Characteristic X-Rays. By measuring those X-Rays very closely we can determine the elements involved and even the orbital from which that hole was filled. By stealing another orbital's electron, there now remains a hole in that orbital which must be filled, and so on. Orbitals in XRF work are labeled according to letters, starting with K for the innermost. Other branches fs science label those same orbitals with numbers, starting with 1. ? As mentioned, this "Fluorescence" of "X-Rays" (XRF) can be causedd by a number of energy sources and carriers. I have discovered that the combination of Gamma Rays and Alpha Particles from @59.5 make a very efficient excitation source. Sometimes only one pellet is needed (<1 uCi) and never have more than 8 spaced around the target been needed. Pictured below is a jig I designed with 8ea. 1/8th" holes into which are inserted the correct # of pellets for a task.Originally designed for the RAP-47 probe, this was called the RAP-CAP. It fits may other probes as you can see. |
||
|
Re: Compare a high end, purpose built NaI(Tl) to an amateur grade Silicon Drift Detector (SDD)
Hi Geo,
toggle quoted message
Show quoted text
That is beautifully clear. I think it is what we are all looking for. Now, I just need to start stuffing that piggy bank a bit harder. Randall ----- Original Message -----
From: GEOelectronics@... To: [email protected] Sent: Fri, 04 Oct 2019 07:38:05 -0700 (PDT) Subject: [XRF] Compare a high end, purpose built NaI(Tl) to an amateur grade Silicon Drift Detector (SDD) This is a scan of a Cesium-Iodide crystal via XRF? that I did in 2013-2014 in Nevada while on a field trip. The portable gamma spec lab was set up on top of our son's pool table in his "Man-Cave". During that trip I did hundreds of scans, both XRF and natural radioactivity of many different samples not to mention a coin collection and just about every inanimate object we could find. Of particular interest was the detailed study of our standard MHO's hardness pint set and out hand cast bullet alloys. . These scans have mostly been in storage since then because the effort was exhausting and other things had to take priority until recently. 3 months of 24 hour scanning using AMPTEK SDD 1-2-3 X-Ray Spectrometer and their free? DppMCA software, Two URSA II? MCA's, and two Spectrum Techniques UCS-20 MCAs.? A wide variety of sodium iodide scintillators was available for individual test setups. Also a wide variety of radioisotopes and one 10 bto 50 keV, energy and power adjustable microfocus X-Ray (most XRF was @ either 10 or 15 microamps) When out in the field collecting samples, the Mobile Rad Lab included two Exploranium GR-135 spectrum analyzers one with the standard internal NaI(Tl), plus internal GM sensors for ratemeter readout, while the other one has the same plus a CZT sensor and a neutron sensor, all internal. The ever present Polimaster PM-1703M pocket alarming pocket detector was utilized in the field as well as the portable Rad Lab, while a much larger Polimaster unit with a larger gamma detector plus He-3 neutron detector was used in the filed with extra external neutron moderators available if needed (they weren't, even at Pantex). Of course several alpha-beta-gamma pancake probe instruments were always at hand. Here's the SDD XRF scan of CsI crystal. More pictures and info will be added as I sift through this mountain of data and photographs. Have fun Geo>K0FF |
||
|
Compare a high end, purpose built NaI(Tl) to an amateur grade Silicon Drift Detector (SDD)
This is a scan of a Cesium-Iodide crystal via XRF? that I did in 2013-2014 in Nevada while on a field trip.
The portable gamma spec lab was set up on top of our son's pool table in his "Man-Cave". During that trip I did hundreds of scans, both XRF and natural radioactivity of many different samples not to mention a coin collection and just about every inanimate object we could find. Of particular interest was the detailed study of our standard MHO's hardness pint set and out hand cast bullet alloys. . These scans have mostly been in storage since then because the effort was exhausting and other things had to take priority until recently. 3 months of 24 hour scanning using AMPTEK SDD 1-2-3 X-Ray Spectrometer and their free? DppMCA software, Two URSA II? MCA's, and two Spectrum Techniques UCS-20 MCAs.? A wide variety of sodium iodide scintillators was available for individual test setups. Also a wide variety of radioisotopes and one 10 bto 50 keV, energy and power adjustable microfocus X-Ray (most XRF was @ either 10 or 15 microamps) When out in the field collecting samples, the Mobile Rad Lab included two Exploranium GR-135 spectrum analyzers one with the standard internal NaI(Tl), plus internal GM sensors for ratemeter readout, while the other one has the same plus a CZT sensor and a neutron sensor, all internal. The ever present Polimaster PM-1703M pocket alarming pocket detector was utilized in the field as well as the portable Rad Lab, while a much larger Polimaster unit with a larger gamma detector plus He-3 neutron detector was used in the filed with extra external neutron moderators available if needed (they weren't, even at Pantex). Of course several alpha-beta-gamma pancake probe instruments were always at hand. Here's the SDD XRF scan of CsI crystal. More pictures and info will be added as I sift through this mountain of data and photographs. Have fun Geo>K0FF |
||
|
Si-PIN low energy Gamma-X-Ray detector upgrade at the Home Lab.
Recent additions to the Home Lab low energy gamma spec lineup now includes a thermoelectrically cooled Amptek Si-PIN detector with 1 mil Be window. Usable range? is from 1 to about 45 keV, covering the area of most interest when studying K,? L and M shell X-Ray fluorescence.
Since the range is limited to 45 keV, this makes Am @ 59.5 the ideal excitation source. I use either a single 0.7uCi centered or 8 X 0.7 uCi in an annular ring. In ne of the first experiments this was used to examine the impact of the alpha particle emission from the excitation source on the observed scan patterns. This picture shows the 300 second scan with the detector and RAPCAP being filtered by adding a single piece of 3x5 paper card stock immediately in front of the RAPCAP. Paper does not have much stopping power @59.5 but has 100% stopping power for alpha particles. (Not much to see) Next the scan is repeated, again for 300 seconds and all other setting exactly the same, but with the paper removed. At this time the sensor remains covered in its own protective plastic cap, so low end energies are being somewhat filtered by that plus the extra distance of air between target and sensor. The "target" here is air, i.e. no target. Analysis follows picture (lots to see): What we see at the low end is alpha particle excited air XRF, specifically Argon, some O and N. Have fun Geo>K0FF |
||
|
Re: MIT XRF experiment with mention of alpha particle calibration XRF energy generator
toggle quoted message
Show quoted text
On 2019/10/02 10:41 AM, Charles David Young wrote:
|
||
|
Re: MIT XRF experiment with mention of alpha particle calibration XRF energy generator
So, George, do you think that if I had, say, 10 Am241 buttons mounted on a lead ring as described in this article that it would be in any way equivalent to an xray tube in terms of exciting XRF in my minerals and competing with the internal radiation?? Of course, I know I need a detector with more resolution in the low energy xray area as well. Charles On Wed, Oct 2, 2019 at 8:26 AM <GEOelectronics@...> wrote: Attached is a page from Glenn F. Knoll's |
||
|
Re: MIT XRF experiment with mention of alpha particle calibration XRF energy generator
Attached is a page from Glenn F. Knoll's
"Radiation Detection and Measurement". I ran across this about 18 years ago and have used it in practice many times since. Eventually this little article morphed into my interest in XRF and the development of the RAPCAP etc. Have Fun Geo>K0FF |
||
|
XRD: X-Ray Diffraction, another Non-Destructive Testing tool.
ntroduction to XRD:
X-ray Powder Diffraction (XRD) Barbara L Dutrow, Louisiana State University , Christine M. Clark, Eastern Michigan University What is X-ray Powder Diffraction (XRD) X-ray powder diffraction (XRD) is a rapid analytical technique primarily used for phase identification of a crystalline material and can provide information on unit cell dimensions. The analyzed material is finely ground, homogenized, and average bulk composition is determined. Fundamental Principles of X-ray Powder Diffraction (XRD) Max von Laue, in 1912, discovered that crystalline substances act as three-dimensional diffraction gratings for X-ray wavelengths similar to the spacing of planes in a crystal lattice. X-ray diffraction is now a common technique for the study of crystal structures and atomic spacing. X-ray diffraction is based on constructive interference of monochromatic X-rays and a crystalline sample. These X-rays are generated by a cathode ray tube, filtered to produce monochromatic radiation, collimated to concentrate, and directed toward the sample. The interaction of the incident rays with the sample produces constructive interference (and a diffracted ray) when conditions satisfy (nλ=2d sin θ). This law relates the wavelength of electromagnetic radiation to the diffraction angle and the lattice spacing in a crystalline sample. These diffracted X-rays are then detected, processed and counted. By scanning the sample through a range of 2θangles, all possible diffraction directions of the lattice should be attained due to the random orientation of the powdered material. Conversion of the diffraction peaks to d-spacings allows identification of the mineral because each mineral has a set of unique d-spacings. Typically, this is achieved by comparison of d-spacings with standard reference patterns. All diffraction methods are based on in an X-ray tube. These X-rays are directed at the sample, and the diffracted rays are collected. A key component of all diffraction is the angle between the incident and diffracted rays. Powder and single crystal diffraction vary in instrumentation beyond this. X-ray Powder Diffraction (XRD) Instrumentation - How Does It Work? X-ray diffractometers consist of three basic elements: an X-ray tube, a sample holder, and an X-ray detector. Bruker's X-ray Diffraction D8-Discover instrument. in a cathode ray tube by heating a filament to produce electrons, accelerating the electrons toward a target by applying a voltage, and bombarding the target material with electrons. When electrons have sufficient energy to dislodge inner shell electrons of the target material, characteristic X-ray spectra are produced. These spectra consist of several components, the most common being Kα and Kβ. Kα consists, in part, of Kα1 and Kα2. Kα1 has a slightly shorter wavelength and twice the intensity as Kα2. The specific wavelengths are characteristic of the target material (Cu, Fe, Mo, Cr). Filtering, by foils or crystal monochrometers, is required to produce monochromatic X-rays needed for diffraction. Kα1and Kα2 are sufficiently close in wavelength such that a weighted average of the two is used. Copper is the most common target material for single-crystal diffraction, with CuKα radiation = 1.5418?. These X-rays are collimated and directed onto the sample. As the sample and detector are rotated, the intensity of the reflected X-rays is recorded. When the geometry of the incident X-rays impinging the sample satisfies the Bragg Equation, constructive interference occurs and a peak in intensity occurs. A detector records and processes this X-ray signal and converts the signal to a count rate which is then output to a device such as a printer or computer monitor.
The geometry of an X-ray diffractometer is such that the sample rotates in the path of the collimated X-ray beam at an angle θ while the X-ray detector is mounted on an arm to collect the diffracted X-rays and rotates at an angle of 2θ. The instrument used to maintain the angle and rotate the sample is termed a goniometer. For typical powder patterns, data is collected at 2θ from ~5° to 70°, angles that are preset in the X-ray scan. Applications X-ray powder diffraction is most widely used for the identification of unknown crystalline materials (e.g. minerals, inorganic compounds). Determination of unknown solids is critical to studies in geology, environmental science, material science, engineering and biology. Other applications include:
With specialized techniques, XRD can be used to:
Strengths and Limitations of X-ray Powder Diffraction (XRD)? Strengths
Limitations
User's Guide - Sample Collection and Preparation Determination of an unknown requires: the material, an instrument for grinding, and a sample holder.
Packing of fine powder into a sample holder.
Typically the substrate is amorphous to avoid interference
·? For unit cell determinations, a small amount of a standard with known peak positions (that do not interfere with the sample) can be added and used to correct peak positions. Data Collection, Results and Presentation Data Collection The intensity of diffracted X-rays is continuously recorded as the sample and detector rotate through their respective angles. A peak in intensity occurs when the mineral contains lattice planes with d-spacings appropriate to diffract X-rays at that value of θ. Although each peak consists of two separate reflections (Kα1 and Kα2), at small values of 2θ the peak locations overlap with Kα2 appearing as a hump on the side of Kα1. Greater separation occurs at higher values of θ. Typically these combined peaks are treated as one. The 2λ position of the diffraction peak is typically measured as the center of the peak at 80% peak height. Data Reduction Results are commonly presented as peak positions at 2θ and X-ray counts (intensity) in the form of a table or an x-y plot (shown above). Intensity (I) is either reported as peak height intensity, that intensity above background, or as integrated intensity, the area under the peak. The relative intensity is recorded as the ratio of the peak intensity to that of the most intense peak (relative intensity = I/I1 x 100 ). Determination of an Unknown The d-spacing of each peak is then obtained by solution of the Bragg equation for the appropriate value of λ. Once all d-spacings have been determined, automated search/match routines compare the ds of the unknown to those of known materials. Because each mineral has a unique set of d-spacings, matching these d-spacings provides an identification of the unknown sample. A systematic procedure is used by ordering the d-spacings in terms of their intensity beginning with the most intense peak. Files of d-spacings for hundreds of thousands of inorganic compounds are available from the as the Powder Diffraction File (PDF). Many other sites contain d-spacings of minerals such as the . Commonly this information is an integral portion of the software that comes with the instrumentation. Determination of Unit Cell Dimensions For determination of unit cell parameters, each reflection must be indexed to a specific hkl. Literature The following literature can be used to further explore X-ray Powder Diffraction (XRD)
Related Links For more information about X-ray Powder Diffraction (XRD) follow the links below.
Teaching Activities and Resources Teaching activities, labs, and resources pertaining to X-ray Powder Diffraction (XRD).
? ? ? ? Pages You Might Like
LInk to above page: |
||
|
Using the Proportional Tube Detector with Beryllium Window for XRF.
开云体育by ? Tue Jul 24, 2012 8:15 pm RE: Conventions used:
Gammas and X-Rays are both electromagnetic radiation and are identical except for their origin. If the photon come from the nucleus it is called a gamma, if it comes from the electron shell area it is called an X-Ray. Other types of photons such as Bremsstrahlung and Annihilation have other genesis mechanisms. Since in this report we are first and foremost interested in the energy of the photon, not it's origin, we call them all 'photons' for simplicity. Be aware that once the energy is calculated, the true nature may then be surmised, then proper name applied to that photon. Some fusioneers are doing neutron activation experiments, the proof of which involves gamma spectroscopy. I thought I would share my recent efforts in 2-50 keV low energy detection/identification. Most of us use NaI(Tl) scintillation detectors for energy studies, those are effective for mid to higher energies (50keV - 3 MeV) A a typical NaI(Tl) is blind to the low energy realm not because of the crystal's ability to respond, but because the crystal is fragile and hygroscopic so must be enclosed in a robust housing to protect it. This robustness of housing material introduces enough mass between the source and crystal to block the lowest energy spectrum. Response of NaI(Tl) is highly dependant on energy, and not in a linear fashion.This phenomena is well studied and all commercial probes have charts available delineating the response at different energies. In the present application, the interest is identifying the energy, not necessarily quantifying it. A typical stainless steel enclosure is good to only around >30 keV, while an aluminum housing can see down to about 10-20 keV. Beryllium can pass 2-3 keV easily, depending on the thickness of the window. Certain experiments involve the photon spectrum well below 20 keV, so special housings and detectors are in order. Types of commercial, readily available detectors: LEG or Low Energy Gamma scintillation probes are indeed made with thin entrance windows, which can pass the required spectrum. Generally these are made of thin section NaI(Tl), so thin it has little or no response to energies above about 100 keV at all. Typical dimensions are 1 or 2 inches diameter and 1 or 2 mm thick. Special versions are available called FIDLERs to 5 inches diameter. Window material varies according to price, with beryllium the preferred but most costly. Such very low Z and thin windows are sometimes protected with a layer of Kapton for strength. Some examples are; Ludlum 44-3. 1" X 1 mm 10-50 keV Ludlum 44-17 2" X 2 mm 10-200 keV Thermo G5 FIDLER (Field Instrument for Detection of Low Energy Radiation) 5" X 1.6 mm >10 keV Bicron 1XM.040BP-X / a.k.a. Canberra Model 1701 1" X 1mm, Be windowed, integral collimator slit. 3-100 keV for Be window, 10-100 keV for aluminum window versions. Typical applications for thin section NaI(Tl) probes are EDS (Energy Dispersive X-Ray Spectroscopy), XRF (X-Ray Fluorescent spectroscopy) and detection of the presence of plutonium (17 keV L X-Ray et. al.), I-125 (27-35 keV), and Am-241 (4 to 59.5 keV). Many varieties and brands of these have been tested at the Home Lab and by and large I do not get an adequate spectrum from them. Detection yes, good spectrum no. The ideal thickness for obtaining a good resolution LE spectrum appears to be about 3-4 mm. I made such a probe based upon the surplus DT-590A probe with very good results. One advantage to exploring the very low energy realm is that little or no shielding is needed over the probe. 1/4" is fine for the thicker of the sodium iodide probes mentioned, while the thinner ones don't really benefit from any shielding at all. The Gas Filled Proportional Counter: Proportional counters are a different beast entirely. Labs use them routinely to make extraordinarily low energy, high resolution measurements. In that application, gas-flow devices are employed, requiring a tank of pressurized "nuclear counting gas" be available, often the P-10 variety containing 90% Argon and 10% Methane. Most of these employ true gas-flow, in which a continuous stream of gas is fed through the probe. A few allow the probe to be initially pressurized, then used for a period of time without further gas added. The main application is for beta-gamma detection and discrimination, not something commonly needed in the Home Lab, at least to this extent. There are a few air-proportional probes that work with regular atmospheric air, but this air must be dehumidified, and the HV adjusted per the altitude of use. While this may be within in the reach of the serious Home Lab experimenter, the complexity and cost make these proportional type systems prohibitive for the student. Still, there are significant advantages to proportional detectors, mostly in the gamma energy detection and IDENTIFICATION capabilities, down at the extreme low end. So we have decided to concentrate on permanently sealed, heavy gas filled proportional probes with beryllium windows. Their high initial cost is offset by the extremely efficient and above all, SIMPLE implementation. Typical examples are the LND models 45419 (Xenon filled) and 45431 (Krypton filled) and 45152 (Neon/Argon filled), all with beryllium side windows. The instrument for this test is a older model RSG-30A, Xenon filled, side beryllium window with an aluminum end window as well. The side window if preferred for ultra low energy studies as it allows entrance of the lowest energy photon , and selectively applies them to only the mid portion of the anode wire, the so called "sweet spot". While the modern equivalent LND probes will work at least as well, their $1000+ price tag is not as attractive as the $50 surplus price for the RSG-30A. As member here are aware from their He3 proportional neutron detector work, all proportional tubes work in the "proportional region" of the gas ionization curve, with gas fill/pressure combinations requiring generally much higher HV than a typical GM tube. At the same time the recovered pulse is of a much lower Voltage peak than a GM tube, requiring the metering unit to be able to provide up to 2500V DC and to respond to 1 to 2 mV pulses. For LE gamma work we want a fill gas to be as dense as possible, in order to optimize the chance of interaction with the weak photons, hence the choice of Xenon fill. Most if not all instruments will require an external preamp to gather the low Voltage pulses to amplify them for application to the MCA or rate meter. Since we are using the SPECTECH UCS-20 as our MCA, the matching SPECTECH PA-1 preamplifier (slightly modified was chosen. NIM users have a broad range of suitable preamps available to match their existing systems. My application is twofold. First is the examine the incredibly low energy X-Rays given off by stable elements when they are excited by an external energy (X-Rays or electron beams). This <20 keV capability allows examination of the L and M shell X-rays, which are very much lower energy than the K shell X-Rays I've been using. Secondly, a sort of take of of the above reason, is to examine naturally occurring L and M X-Rays emitted by the daughter products of radioactive elements. The first such test was on Am-241, an examination of the Np L and M shell X-Rays. The attached scan shows the result with my notes as to the presumed sources and energies of the photons detected. The unlabeled peak at 8.5 keV has not yet been identified. ADDED 26 July 2012, Energy Response Curve File (picture) George Dowell
|
||
|
Re: Hello! Lixi PS-500 unit
toggle quoted message
Show quoted text
On 2018/10/29 11:22 AM, Nick Andrews wrote:
|
||
|
Re: Hello! Lixi PS-500 unit
Well my new friend just got here!? ?She's a hefty girl.? They did carefully foam it in the box.? ?I pulled every piece for a quick inspection and it looks like it's all there,? if a little dusty/grimy.?? One display panel on the power supply is popped loose from the front panel,? likely from the foam.? Nothing looks like an impact.? The real killer is that I leave for a conference in the morning and need to pack tonight,? back Sunday so I might not get a chance to play with it and maybe power it up until some time next week.? Looks like the little box with knobs is for the ccd camera.? The source/ imager assembly is really heavy,? thick stainless steel.? ?I'm at work and have a ton to do,? so I didn't take any pictures.? Plus,? the office girls up front think I'm a little more crazy than before,? so... On Sun, Oct 21, 2018, 3:47 PM <GEOelectronics@...> wrote: Picture of same model(?) that ran through the shop last year- |
||
|
Re: Charles- cerium in minerals question. Geo
开云体育Congrat's on that astute finding Dave! ?
On 2018/10/21 06:11 PM, David Eckhardt wrote:
|
||
|
Re: Charles- cerium in minerals question. Geo
Self Excitation:? In working with Luuk's new (to me) 2" x 2" CsI 'puck', I was quite surprised to detect self excitation of the Bi, U, and Ra XRF lines using a small chunk (1 x 0.5 x 0.2 cm) of uraninite as the source.? After doing a three-point calibration using both Cs-137 and Am-241 of the low end, the lines were right on.? Of course, I had to 'fine tune' Theremino to bring out the XRF lines, but they were clearly there. ? I haven't yet tried copper with this new probe, but that will be excited with an Am-241 pellet.? Dave - W?LEVOf course, I had to d? ? On Sun, Oct 21, 2018 at 8:59 PM <GEOelectronics@...> wrote: Very interesting Charles. Normally I discount self-XRF ideas but your scans make a compelling argument. Will look into that in future... -- Dave - W?LEV Just Let Darwin WorkJust Think |









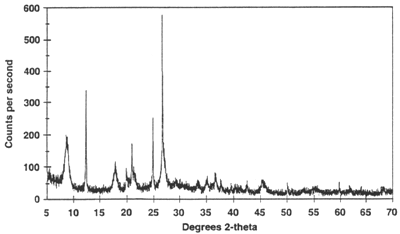 X-ray powder diffractogram. Peak positions occur where the X-ray beam has been diffracted by the crystal lattice. The unique set of d-spacings derived from this patter can be used to 'fingerprint' the mineral.
X-ray powder diffractogram. Peak positions occur where the X-ray beam has been diffracted by the crystal lattice. The unique set of d-spacings derived from this patter can be used to 'fingerprint' the mineral.